Calculate the lattice energy for NaCl(s) using a Born-Haber cycle and the following information: 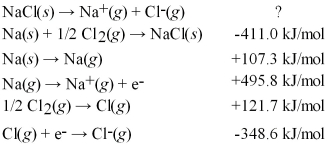
A) +34.8 kJ/mol
B) +690.3 kJ/mol
C) +787.2 kJ/mol
D) +1512 kJ/mol
Correct Answer:
Verified
Q10: List the elements Na,Ca,Rb,Cl,He in order of
Q32: Which of these elements has the most
Q38: Which of the following species will have
Q39: Consider the following electron configurations for neutral
Q40: Which ionization process requires the most energy?
A)P(g)→
Q41: An element M reacts with chlorine to
Q43: To reach a noble gas electron configuration
Q44: Calculate the energy change for the formation
Q45: Calculate the energy change for the formation
Q46: Calculate the energy change for the formation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents