Calculate the energy change for the formation of CaF2(s) from its elements in their standard states and the following information: 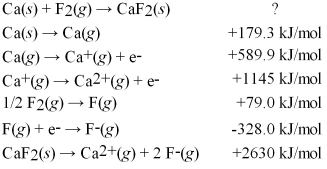
A) +4046 kJ/mol
B) -965 kJ/mol
C) -1214 kJ/mol
D) -3286 kJ/mol
Correct Answer:
Verified
Q28: What is the general trend in ionization
Q32: Which of these elements has the most
Q33: An element that has the valence electron
Q41: An element M reacts with chlorine to
Q42: Calculate the lattice energy for NaCl(s)using a
Q43: To reach a noble gas electron configuration
Q44: Calculate the energy change for the formation
Q45: Calculate the energy change for the formation
Q49: Calculate the energy change in kJ/mol for
Q51: How many electrons does magnesium lose and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents