Multiple Choice
The following drawing is a representation of a reaction for which ΔH° = -22 kJ.This reaction is likely to be 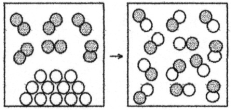
A) nonspontaneous at all temperatures.
B) nonspontaneous at low temperatures and spontaneous at high temperatures.
C) spontaneous at low temperatures and nonspontaneous at high temperatures.
D) spontaneous at all temperatures.
Correct Answer:
Verified
Related Questions
Q62: Consider the conversion of white tin to
Q65: The enthalpy of fusion of naphthalene,C10H8,is 19.1
Q78: When heated,mercury(II)oxide decomposes into elemental mercury and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents