A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp = 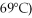 and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 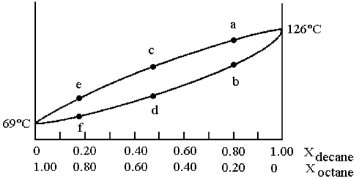
-Assume that you start with a mixture containing 0.80 mol of decane and 0.20 mol of octane,what is the vapor composition at the boiling point?
A) 100% decane
B) composition at point b
C) composition at point c
D) composition at point e
Correct Answer:
Verified
Q97: Arrows in the energy diagram below represent
Q97: Arrows in the energy diagram below represent
Q98: Q100: Q101: A gold ring is an example of Q103: A phase diagram of temperature versus composition Q104: Two beakers,one with pure water (light gray)and Q105: A phase diagram of temperature versus composition Q106: A phase diagram of temperature versus composition Q107: Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

