A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp =  and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 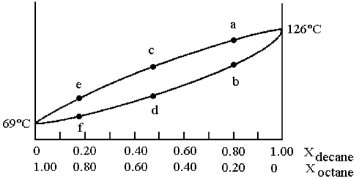
-Assume that the vapor at point c is condensed and reboiled.What is the boiling point?
A) temperature at point b
B) temperature at point c
C) temperature at point d
D) temperature at point f
Correct Answer:
Verified
Q109: A phase diagram of temperature versus composition
Q110: A phase diagram of temperature versus composition
Q111: Q112: At 80°C,pure liquid A has a vapor Q113: KI does not dissolve well in nonpolar Q115: Which cation in each set is expected Q116: In the process of dissolving ionic compounds,the Q117: Which cation in each set would be Q118: Drawing (1)shows a nonequilibrium system comprised of Q119: A phase diagram of temperature versus composition![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents