The decomposition of cyclopropane,was observed at 500°C and its concentration was monitored as a function of time.The data set is given below.What is the order of the reaction with respect to cyclopropane? 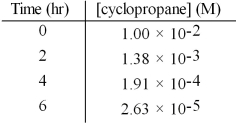
A) zero
B) first
C) second
D) third
Correct Answer:
Verified
Q25: If a sample of 233Pa takes 62.7
Q25: The half life of the reaction shown
Q32: The first-order reaction,SO2Cl2 → SO2 + Cl2,has
Q33: What percentage of a radioactive substance remains
Q39: Fluorine-18 is an isotope used in Positron
Q46: The isomerization reaction,CH3NC → CH3CN,is first order
Q48: Acetaldehyde decomposes at 750 K: CH3CHO →
Q50: The decay constant,k,is given for each of
Q58: For the first-order reaction,2 N2O(g)→ 2 N2(g)+
Q116: The rate constant,k,for a first-order reaction is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents