The following picture represents the equilibrium state for the reaction A2 + B2 ⇌ 2AB.What is the relationship between the rate constant for the forward reaction,kf,and the rate constant for the reverse reaction kr? 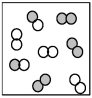
A) kf < kr
B) kf = kr = 0
C) kf = kr
D) kf > kr
Correct Answer:
Verified
Q85: The reaction A2 + B2 ⇌ 2AB
Q90: The following pictures represent the initial state
Q93: Consider the reaction A + B ⇌
Q103: Picture (1)represents an equilibrium mixture of solid
Q115: Shown below is a concentration vs.time plot
Q117: The reaction A2 + B2 ⇌ 2
Q119: The following pictures represent mixtures of A2B4
Q120: The following picture represents the equilibrium state
Q121: At a certain temperature,nitrogen and hydrogen react
Q123: For the reaction: N2(g)+ 2 O2(g)⇌ 2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents