Which metal sulfides can be precipitated from a solution that is 0.01 M in Mn2+,Zn2+,Pb2+ and Cu2+ and 0.10 M in H2S at a pH of 1.0? 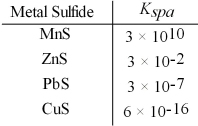
A) MnS
B) CuS
C) PbS,CuS
D) ZnS,PbS,CuS
Correct Answer:
Verified
Q62: The following pictures represent solutions that contain
Q64: A solution may contain the following ions
Q65: 0.10 M potassium chromate is slowly added
Q68: The following pictures represent solutions that contain
Q70: The following pictures represent solutions that contain
Q70: The following pictures represent solutions that contain
Q76: The following pictures represent solutions that contain
Q79: The following pictures represent solutions that contain
Q88: Which metal ions can be precipitated out
Q165: Which set of ions precipitate as sulfides?
A)Ag+,Pb2+,Mn2+
B)Pb2+,Fe2+,Ca2+
C)Co2+,Ba2+,K+
D)NH4+,Na+,K+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents