What is the relationship between the standard cell potentials,E°,for the following two galvanic cell reactions?
I.3 Cu2+(aq) + 2 Al(s) → 3 Cu(s) + 2 Al3+(aq)
II.6 Cu2+(aq) + 4 Al(s) → 6 Cu(s) + 4 Al3+(aq)
A) E°(I) = E°(II)
B) E°(I) = 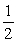 E°(II)
E°(II)
C) E°(I) = 2E°(II)
D) E°(I) = E°(II) 2
Correct Answer:
Verified
Q21: What is the balanced chemical equation for
Q23: For the hypothetical reaction A + Bx
Q29: Write the overall cell reaction for the
Q31: What is the balanced equation for the
Q32: In the relationship ΔG = -nFE°,what is
Q36: Doubling all the coefficients in the equation
Q38: What is the relation between ΔG° and
Q40: For the hypothetical reaction A + 2
Q49: A galvanic cell consists of a La3+/La
Q53: Using the following standard reduction potentials Fe3+(aq)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents