Shown below is a galvanic cell with anode compartment b containing anode a and cathode compartment d containing cathode c.Electrons flow through wire f,ions flow through salt bridge e,and the cell potential is read using voltmeter g.
This galvanic cell uses the reaction: Cu(s) + 2 Ag+(aq) 2 Ag(s) + Cu2+(aq) . 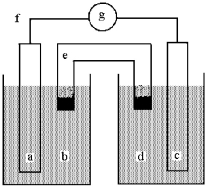
-The initial concentrations of Ag+(aq) and Cu2+(aq) are both 1.0 M.What will happen to the cell voltage if 5.0 M Cu(NO3) 2 is added to the compartment containing the 1.0 M Cu2+(aq) ? The cell voltage will
A) decrease.
B) increase.
C) remain the same.
D) can't tell from the information given
Correct Answer:
Verified
Q101: Shown below is a galvanic cell with
Q103: Shown below is a galvanic cell with
Q112: If the concentrations of Ag+(aq)and Cu2+(aq)are varied
Q119: What is the shorthand notation for this
Q123: The initial concentrations of Ag+(aq)and Cu2+(aq)are both
Q125: Shown below is a galvanic cell
Q129: Consider the galvanic cell shown below.
Q131: What is the reduction half-reaction for the
Q135: Consider the galvanic cell shown below.
Q139: Consider the following galvanic cell. 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents