The following molecular orbital energy level diagram shows the energies and occupancies of the MOs derived from the atomic 2p orbitals for an oxygen-containing binary compound of potassium.This compound is a 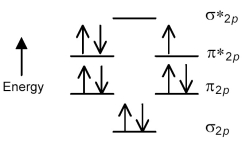
A) peroxide that is attracted by magnetic fields.
B) peroxide that is repelled by magnetic fields.
C) superoxide that is attracted by magnetic fields.
D) superoxide that is repelled by magnetic fields.
Correct Answer:
Verified
Q135: The following molecular orbital energy level diagram
Q136: Look at the location of elements A,B,C,and
Q137: How many liters of hydrogen gas can
Q138: Look at the location of elements A,B,C,and
Q140: What is the total volume of the
Q141: How many milliliters of ozone gas at
Q142: When 1.800 grams of anhydrous magnesium chloride
Q143: When 0.350 grams of anhydrous copper(II)sulfate is
Q144: What is the total volume of hydrogen
Q167: How many grams of H2 gas can
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents