Given:
4AlCl3(s) + 3O2(g) → 2Al2O3(s) + 6Cl2(g) ; ΔH = -529.0 kJ
Determine ΔH for the following thermochemical equation.
Cl2(g) +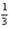 Al2O3(s) →
Al2O3(s) → 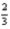 AlCl3(s) +
AlCl3(s) +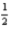 O2(g)
O2(g)
A) +264.5 kJ
B) +529.0 kJ
C) +88.2 kJ
D) +176.3 kJ
E) -176.3 kJ
Correct Answer:
Verified
Q27: Which of the following statements is incorrect
Q28: How much heat is liberated at constant
Q29: According to the following thermochemical equation,if 403.3
Q30: What quantity,in moles,of oxygen is consumed when
Q31: Consider the following thermochemical equation:
N2(g)+ 2O2(g)→ 2NO2(g);
Q33: What is the change in enthalpy at
Q34: In a certain experiment,0.7000 mol of hydrogen
Q35: Which of the following statements is false
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents