Given: 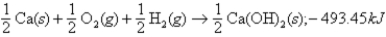 what is ΔH for the following thermochemical equation?
what is ΔH for the following thermochemical equation? 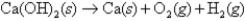
A) 986.9 kJ
B) -986.9 kJ
C) -139 MJ
D) -2320 kJ
E) -38.7 kJ
Correct Answer:
Verified
Q31: Consider the following thermochemical equation:
N2(g)+ 2O2(g)→ 2NO2(g);
Q32: Given:
4AlCl3(s)+ 3O2(g)→ 2Al2O3(s)+ 6Cl2(g); ΔH = -529.0
Q33: What is the change in enthalpy at
Q34: In a certain experiment,0.7000 mol of hydrogen
Q35: Which of the following statements is false
Q37: Which of the following sentences accurately describes
Q38: What is the change in enthalpy at
Q39: Given the thermochemical equation Q40: What mass of oxygen is consumed when Q41: A 3.540-g sample of an unknown metal
2Al(s)+ ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents