Multiple Choice
What mass of oxygen is consumed when 285.5 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g) ?
H2(g) + 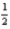
O2(g) → H2O(l) ; ΔH° = -285.8 kJ
A) 15.98 g
B) 31.96 g
C) 0.01564 g
D) 31.98 g
E) 0.01679 g
Correct Answer:
Verified
Related Questions
Q35: Which of the following statements is false
Q36: Given: Q37: Which of the following sentences accurately describes Q38: What is the change in enthalpy at![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents