Using two or more of the following,
N2(g) + 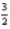
O2(g) → N2O3(s) ; ΔH° = 83.7 kJ
N2(g) + O2(g) → 2NO(g) ; ΔH° = 180.4 kJ 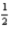
N2(g) + O2(g) → NO2(g) ; ΔH° = 33.2 kJ 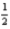
N2(g) + 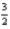
H2(g) → NH3(g) ; ΔH° = −45.9 kJ
Determine ΔH° for the following reaction.
NO(g) + NO2(g) → N2O3(g)
A) -39.7 kJ
B) 24.3 kJ
C) -207.1 kJ
D) 39.7 kJ
E) 207.1 kJ
Correct Answer:
Verified
Q80: Which of the following processes will result
Q81: Which of the following species does not
Q82: All of the following have a standard
Q83: The balanced equation representing the standard enthalpy
Q84: Which of the following reactions corresponds to
Q86: The enthalpy change at 1 atm of
Q87: What is ΔH° for the following phase
Q88: Which of the following has a standard
Q89: Given that
O(g)+ e- → O-(g); ΔH =
Q90: Consider the following changes at constant temperature
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents