Which of the following reactions corresponds to the thermochemical equation for the standard enthalpy of formation of N,N-diethyl-m-toluamide,C12H17NO(l) ,the active ingredient in some insect repellents?
A) 12C(l) + 17H(l) + N(l) + O(l) → C12H17NO(l)
B) 12C(g) + 17H(g) + N(g) + O(g) → C12H17NO(g)
C) 12C(s) + 17H(g) + N(g) + O(g) → C12H17NO(l)
D) 12C(s) + 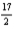 H2(g) +
H2(g) + 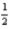 N2(g) +
N2(g) + 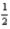 O2(g) → C12H17NO(l)
O2(g) → C12H17NO(l)
E) 12C(g) + 17H(g) + N(g) + O(g) → C12H17NO(l)
Correct Answer:
Verified
Q79: Combustion of 3.65 g of liquid benzene
Q80: Which of the following processes will result
Q81: Which of the following species does not
Q82: All of the following have a standard
Q83: The balanced equation representing the standard enthalpy
Q85: Using two or more of the following,
N2(g)+
Q86: The enthalpy change at 1 atm of
Q87: What is ΔH° for the following phase
Q88: Which of the following has a standard
Q89: Given that
O(g)+ e- → O-(g); ΔH =
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents