Combustion of 3.65 g of liquid benzene (C6H6) causes a temperature rise of 21.1°C in a constant-pressure calorimeter that has a heat capacity of 7.23 kJ/°C.What is ΔH for the following reaction?
C6H6(l) + 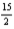 O2(g) → 6CO2(g) + 3H2O(l)
O2(g) → 6CO2(g) + 3H2O(l)
A) 150 kJ/mol
B) 41.8 kJ/mol
C) -41.8 kJ/mol
D) -3.27× 103 kJ/mol
E) 152 kJ/mol
Correct Answer:
Verified
Q74: In a bomb calorimeter,reactions are carried out
A)at
Q75: A bomb calorimeter has a heat capacity
Q76: Given:
Pb(s)+ PbO2(s)+ 2H2SO4(l)→ 2PbSO4(s)+ 2H2O(l); ΔH° =
Q77: Using the following data,calculate the standard enthalpy
Q78: The overall chemical equation resulting from the
Q80: Which of the following processes will result
Q81: Which of the following species does not
Q82: All of the following have a standard
Q83: The balanced equation representing the standard enthalpy
Q84: Which of the following reactions corresponds to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents