A bomb calorimeter has a heat capacity of 2.47 kJ/K.When a 0.106-g sample of a certain hydrocarbon was burned in this calorimeter,the temperature increased by 2.14 K.Calculate the energy of combustion for 1 g of the hydrocarbon.
A) -5.29 J/g
B) 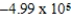 J/g
J/g
C) -0.120 J/g
D) 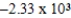 J/g
J/g
E) -0.560 J/g
Correct Answer:
Verified
Q70: How much heat is gained by copper
Q71: When 9.42 g of methane (CH4)is burned
Q72: When 50.0 mL of 1.27 M of
Q73: A 86.9-g sample of chromium (s =
Q74: In a bomb calorimeter,reactions are carried out
A)at
Q76: Given:
Pb(s)+ PbO2(s)+ 2H2SO4(l)→ 2PbSO4(s)+ 2H2O(l); ΔH° =
Q77: Using the following data,calculate the standard enthalpy
Q78: The overall chemical equation resulting from the
Q79: Combustion of 3.65 g of liquid benzene
Q80: Which of the following processes will result
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents