What is the standard enthalpy of formation of liquid methylamine (CH3NH2) ?
C(s) + O2(g) → CO2(g) ; ΔH° = -393.5 kJ
2H2O(l) → 2H2(g) + O2(g) ; ΔH° = 571.6 kJ 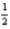
N2(g) + O2(g) → NO2(g) ; ΔH° = 33.10 kJ
4CH3NH2(l) + 13O2(g) → 4CO2(g) + 4NO2(g) + 10H2O(l) ; ΔH° = -4110.4 kJ
A) +3899.2 kJ/mol
B) -3899.2 kJ/mol
C) -47.3 kJ/mol
D) +47.3 kJ/mol
E) +3178.4 kJ
Correct Answer:
Verified
Q93: Which of the following has a standard
Q94: The standard enthalpy change for which of
Q95: For which of the following equations is
Q96: Which of the following reactions corresponds to
Q97: Given the following thermochemical data at 25°C
Q99: Which substance has a standard enthalpy of
Q100: Given the following data,calculate the standard enthalpy
Q101: What is the standard enthalpy of formation
Q102: What is the standard enthalpy of formation
Q103: Which of the following is not a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents