Given the following thermochemical data at 25°C and 1 atm pressure, 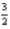 O2(g) + 2B(s) → B2O3(s) ; ΔH° = -1264 kJ
O2(g) + 2B(s) → B2O3(s) ; ΔH° = -1264 kJ
O3(g) + 2B(s) → B2O3(s) ; ΔH° = -1406 kJ
Determine ΔH° for the following reaction at 25°C and 1 atm pressure.
3O2(g) → 2O3(g)
A) -980 kJ/mol
B) +284 kJ/mol
C) +980 kJ/mol
D) -2670 kJ/mol
E) -284 kJ/mol
Correct Answer:
Verified
Q92: The enthalpy change at 1 atm of
Q93: Which of the following has a standard
Q94: The standard enthalpy change for which of
Q95: For which of the following equations is
Q96: Which of the following reactions corresponds to
Q98: What is the standard enthalpy of formation
Q99: Which substance has a standard enthalpy of
Q100: Given the following data,calculate the standard enthalpy
Q101: What is the standard enthalpy of formation
Q102: What is the standard enthalpy of formation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents