The enthalpy change at 1 atm of which reaction corresponds to the standard enthalpy of formation of solid potassium bromate,KBrO3?
A) K(s) + Br(g) + 3O(g) → KBrO3(s)
B) K(g) + Br(g) + 3O(g) → KBrO3(s)
C) K(g) + 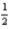 Br2(g) +
Br2(g) + 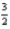 O2(g) → KBrO3(s)
O2(g) → KBrO3(s)
D) K(s) + 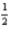 Br2(l) +
Br2(l) + 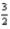 O2(g) → KBrO3(s)
O2(g) → KBrO3(s)
E) K(s) + 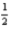 Br2(g) +
Br2(g) + 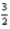 O2(g) → KBrO3(s)
O2(g) → KBrO3(s)
Correct Answer:
Verified
Q87: What is ΔH° for the following phase
Q88: Which of the following has a standard
Q89: Given that
O(g)+ e- → O-(g); ΔH =
Q90: Consider the following changes at constant temperature
Q91: Which of the following has a standard
Q93: Which of the following has a standard
Q94: The standard enthalpy change for which of
Q95: For which of the following equations is
Q96: Which of the following reactions corresponds to
Q97: Given the following thermochemical data at 25°C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents