The Arrhenius equation, 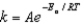 expresses the dependence of the rate constant on the reaction temperature.The slope of a plot of ln(k) versus 1/T is equal to
expresses the dependence of the rate constant on the reaction temperature.The slope of a plot of ln(k) versus 1/T is equal to
A) 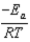
B) 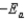
C) 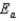
D) 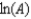
E) 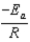
Correct Answer:
Verified
Q83: Which of the following statements is not
Q84: A mechanism that explains the rate law,Rate
Q85: Given the following fast equilibrium (below)proposed for
Q86: Below is a proposed mechanism for the
Q87: Which of the following statements is incorrect?
A)The
Q89: The following mechanism has been suggested for
Q90: The complete mechanism for a reaction is
Q91: The rate constants for the first-order decomposition
Q92: A proposed mechanism for the decomposition of
Q93: A proposed mechanism for the decomposition of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents