A suggested mechanism for the decomposition of ozone is
O3 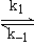
O2 + O fast equilibrium
O + O3 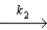
2O2 slow step
When the concentration of ozone is doubled and the concentration of oxygen is doubled,the instantaneous rate
A) increases by a factor of 8.
B) remains the same.
C) increases by a factor of 4.
D) decreases.
E) increases by a factor of 2.
Correct Answer:
Verified
Q102: In a chemical reaction at constant temperature,the
Q103: The rate constant for a first-order reaction
Q104: Which of the following reactions is not
Q105: In the Arrhenius equation,the symbol A is
Q106: Which of the following reactions is not
Q108: The catalyzed pathway in a reaction mechanism
Q109: Identify the Arrhenius equation from the following.
A)
Q110: The rate law for a reaction is
Q111: Identify the rate equation of the
Q112: Which of the following statements is incorrect
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents