Which of the following reactions is not an example of heterogeneous catalysis?
A) CO(g) + 3H2(g) 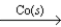 CH4(g) + H2O(g)
CH4(g) + H2O(g)
B) C2H4(g) + H2(g) 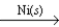 C2H6(g)
C2H6(g)
C) 2CO(g) + 2NO(g) 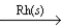 2CO2(g) + N2(g)
2CO2(g) + N2(g)
D) 2O3(g) 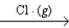 3O2(g)
3O2(g)
E) 2SO2(g) + O2(g) 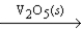 2SO3(g)
2SO3(g)
Correct Answer:
Verified
Q99: The decomposition of ozone may occur through
Q100: The reaction CHCl3(g)+ Cl2(g) Q101: How many mechanistic steps are depicted by Q102: In a chemical reaction at constant temperature,the Q103: The rate constant for a first-order reaction Q105: In the Arrhenius equation,the symbol A is Q106: Which of the following reactions is not Q107: A suggested mechanism for the decomposition of Q108: The catalyzed pathway in a reaction mechanism Q109: Identify the Arrhenius equation from the following.![]()
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents