Which of the following is always true for a reaction where Kc is 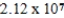 at 25°C?
at 25°C?
A) The reaction mixture contains mostly products at equilibrium.
B) The reaction mixture contains mostly reactants at equilibrium.
C) The rate of reaction is very fast.
D) There are approximately equal moles of reactants and products at equilibrium.
E) Both A and C.
Correct Answer:
Verified
Q29: For which of the following reactions will
Q30: What is the Kc expression for the
Q31: At 298 K,the value of Kc for
Q32: Given the equilibrium constants for the equilibria,
22NH4+(aq)+
Q33: For which of the following values of
Q35: Which of the following is true for
Q36: What is the Kc equilibrium-constant expression for
Q37: Consider the following reaction: Q38: Consider the following equilibrium: Q39: For which of the following equilibria does
2HF(g) ![]()
O2(g)+ 2F2(g) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents