The equilibrium hydronium ion concentration of an initially 0.655 M solution of a monoprotic weak acid is  M.The acid dissociation constant is
M.The acid dissociation constant is 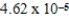
At 25°C.What is the pH of this solution?
A) 2.26
B) 7.00
C) 11.74
D) 4.52
E) 0.18
Correct Answer:
Verified
Q35: Which of the following represents the usual
Q36: It is safe to make the simplifying
Q37: What is the percent ionization of a
Q38: For a 0.10 M solution of glutaric
Q39: What is the hydronium-ion concentration of a
Q41: What is the hydronium-ion concentration of a
Q42: Which of the following reactions is associated
Q43: What is the pH of a 0.35
Q44: What is the concentration of C2O42- in
Q45: What is the concentration of HC2O4- in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents