Rank the following salts in order of increasing molar solubility.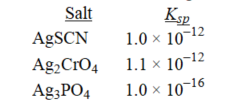
A) AgSCN < Ag2CrO4 < Ag3PO4
B) AgSCN < Ag3PO4 < Ag2CrO4
C) Ag3PO4 < Ag2CrO4 < AgSCN
D) Ag3PO4 < AgSCN < Ag2CrO4
E) Ag2CrO4 < AgSCN < Ag3PO4
Correct Answer:
Verified
Q42: What is the pH of a saturated
Q43: Which of the following will apply to
Q44: For which of the following will precipitation
Q45: What is the concentration of silver(I)ion in
Q46: To 1.0 L of water,1.5 × 10-6
Q48: The solubility of La(IO3)3 in a 0.71
Q49: The insoluble salts AV,B2W,C2X3,DY2,and EZ3,which were formed
Q50: Ksp for PbF2 is 4.0 ×10-8.If a
Q51: What is the hydroxide-ion concentration of a
Q52: Suppose 50.00 mL of 2.0 × 10-6
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents