What is the maximum concentration of carbonate ions that will precipitate BaCO3 but not MgCO3 from a solution that is 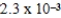 M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 × 10-5 and for BaCO3,Ksp = 2.6 × 10-9.
M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 × 10-5 and for BaCO3,Ksp = 2.6 × 10-9.
A) 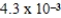 M
M
B) 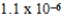 M
M
C) 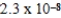 M
M
D) 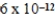 M
M
E) 2.6 × 10-14 M
Correct Answer:
Verified
Q58: Which salt has the lowest molar solubility
Q59: In which of the following solutions would
Q60: What is the molar solubility of MgF2
Q61: A 5.0 × 10-4 M solution of
Q62: For which pair of cations would the
Q64: If 275 mL of 1 × 10-7
Q65: What is the maximum hydroxide-ion concentration that
Q66: If 370 mL of 1 × 10-8
Q67: What is the minimum mass of Na2CO3
Q68: Suppose 50.00 mL of a 1 ×
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents