Cyanide ion forms very stable complex ions with a variety of metal ions.What is the molar equilibrium concentration of uncomplexed Ni2+(aq) in a solution that initially contains 1.3 mol of Ni(CN) 42− per liter of solution .Kf for Ni(CN) 42− is  .
.
A)  M
M
B) 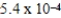 M
M
C) 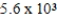 M
M
D) 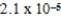 M
M
E) 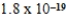 M
M
Correct Answer:
Verified
Q82: For which of the following salts would
Q83: The figure below represents the results of
Q84: Suppose hydrogen sulfide is added to a
Q85: Which sparingly soluble salt will exhibit the
Q86: You have two salts,AgX and AgY,with very
Q88: Which of the following is not likely
Q89: Calculate the molar concentration of uncomplexed Zn2+(aq)in
Q90: Suppose sodium hydroxide is added to a
Q91: Which of the following substances will increase
Q92: Suppose hydrogen sulfide is added to a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents