Calculate the molar concentration of uncomplexed Zn2+(aq) in a solution that contains 0.21 mol of Zn(NH3) 42+ per liter and 0.4986 M NH3 at equilibrium.Kf for Zn(NH3) 42+ is 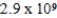 .
.
A) 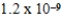 M
M
B) 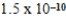 M
M
C) 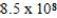 M
M
D) 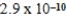 M
M
E) 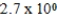 M
M
Correct Answer:
Verified
Q84: Suppose hydrogen sulfide is added to a
Q85: Which sparingly soluble salt will exhibit the
Q86: You have two salts,AgX and AgY,with very
Q87: Cyanide ion forms very stable complex ions
Q88: Which of the following is not likely
Q90: Suppose sodium hydroxide is added to a
Q91: Which of the following substances will increase
Q92: Suppose hydrogen sulfide is added to a
Q93: What is the molar equilibrium concentration of
Q94: The figure below represents the results of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents