What is the molar equilibrium concentration of uncomplexed Fe2+(aq) in a solution composed of 1.4 mol Fe(CN) 64− dissolved in 1.00 L of 0.33 M NaCN.Kf for Fe(CN) 64− is 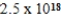 .
.
A) 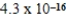 M
M
B) 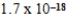 M
M
C) 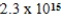 M
M
D) 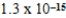 M
M
E) 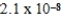 M
M
Correct Answer:
Verified
Q88: Which of the following is not likely
Q89: Calculate the molar concentration of uncomplexed Zn2+(aq)in
Q90: Suppose sodium hydroxide is added to a
Q91: Which of the following substances will increase
Q92: Suppose hydrogen sulfide is added to a
Q94: The figure below represents the results of
Q95: An aqueous solution of Ag(CN)2− is made
Q96: What is the concentration of Cd2+ in
Q97: The best explanation for the dissolution of
Q98: In which of the following solutions would
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents