If the cell is initially at standard-state conditions,which of the following statements is true? 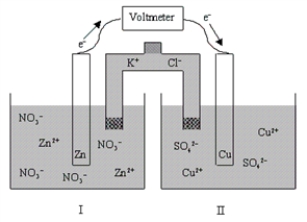 Zn2+(aq) + 2e-
Zn2+(aq) + 2e-  Zn(s) ; E° = -0.76 V
Zn(s) ; E° = -0.76 V
Cu2+(aq) + 2e-  Cu(s) ; E° = 0.34 V
Cu(s) ; E° = 0.34 V
A) Initially 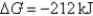 ,and it will become more positive with time.
,and it will become more positive with time.
B) Initially 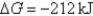 ,and it will not change with time.
,and it will not change with time.
C) Initially 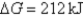 ,and it will become more negative with time.
,and it will become more negative with time.
D) Initially 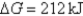 ,and it will become more positive with time.
,and it will become more positive with time.
E) Initially 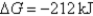 ,and it will become more negative with time.
,and it will become more negative with time.
Correct Answer:
Verified
Q58: Given: Q59: Given: Q60: Given: Q61: Given: Q62: The cell potential of the following cell Q64: What is the logarithm of the equilibrium Q65: The cell potential of the following cell Q66: Calculate the maximum electrical work obtainable at Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents
Li+(aq)+ e- ![]()
2H+(aq)+ 2e- ![]()
Zn2+(aq)+ 2e- ![]()
![]()

