Multiple Choice
Given: 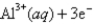

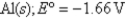
Cl2 (g) + 2e¯  2Cl¯ (aq) ; Eº = 1.36V
2Cl¯ (aq) ; Eº = 1.36V
What is 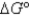
For the following cell reaction?
2AlCl3(aq)  2Al(s) + 3Cl2(g)
2Al(s) + 3Cl2(g)
A) -5.8 × 105 J
B) 5.8 × 105 J
C) 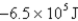
D) -1.7 × 106 J
E) 1.7 × 106 J
Correct Answer:
Verified
Related Questions
Q56: Consider the following reduction potentials:
Cd2+(aq)+ 2e-
Q57: Which of the following is true for

