What is the value of the reaction quotient,Q,for the voltaic cell constructed from the following two half-reactions when the Zn2+ concentration is 0.0120 M and the Ag+ concentration is 1.27 M? 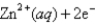

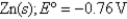
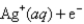

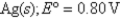
A) 9.45 × 10-3
B) 7.44 × 10-3
C) 106
D) 1.25 × 10-2
E) 134
Correct Answer:
Verified
Q90: In order to determine the identity of
Q91: What half-reaction occurs at the cathode during
Q92: Cathodic protection results when
A)iron is amalgamated with
Q93: A piece of iron half-immersed in a
Q94: A cell consists of a magnesium electrode
Q96: The following cell is initially at standard-state
Q97: Which of the following statements is true
Q98: For the cell reaction
2MnO4- (aq)+ 5SO2(g)+ 2H2O(l)
Q99: What is the reduction potential for the
Q100: A voltaic cell is made by placing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents