A solution of 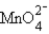 is electrolytically reduced to Mn3+.A current of 5.19 A is passed through the solution for 15.0 min.What is the number of moles of Mn3+ produced in this process? (1 faraday = 96,486 coulombs)
is electrolytically reduced to Mn3+.A current of 5.19 A is passed through the solution for 15.0 min.What is the number of moles of Mn3+ produced in this process? (1 faraday = 96,486 coulombs)
A) 0.145 mol
B) 1.08 × 10-3 mol
C) 0.0161 mol
D) 0.0484 mol
E) 2.69 × 10-4 mol
Correct Answer:
Verified
Q112: How many faradays are required to convert
Q113: When an aqueous solution of sodium sulfate
Q114: Gold (atomic weight = 197)is plated from
Q115: What reaction occurs at the anode during
Q116: Electrolysis of a molten salt with the
Q117: Which of the following statements is true
Q118: What is the half-reaction that occurs at
Q120: An alkaline dry cell is similar to
Q121: An amalgam is an alloy of _
Q122: A _ is a commercial electrochemical cell
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents