In an isothermal process where the pressure decreases from 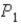
To 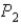
,does the internal energy of the system increase,decrease,or stay the same;and does the entropy of the system increase or decrease?
A) The internal energy decreases and the entropy increases.
B) The internal energy stays the same and the entropy increases.
C) The internal energy decreases and the entropy decreases.
D) The internal energy stays the same and the entropy decreases.
Correct Answer:
Verified
Q23: A gasoline engine with an efficiency of
Q26: An avalanche of ice and snow of
Q28: During each cycle of operation a refrigerator
Q29: A cylinder containing an ideal gas has
Q31: When gasoline is burned,it gives off 46,000
Q32: A 600-MW electric power plant has an
Q61: You have a PV diagram for a
Q67: Consider a Carnot cycle starting at the
Q87: The laws of thermodynamics can be related
Q89: The laws of thermodynamics can be related
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents