Sample #1 is made from an isotope with decay constant 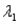
And sample #2 is made from an isotope with decay constant 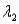
,where 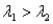
) Which of the following statements must be true?
A) The activity of sample #2 is greater than that of sample #1.
B) The activity of sample #1 is greater than that of sample #2.
C) The half-life exhibited for sample #1 is greater than that of sample #2.
D) The half-life exhibited for sample #2 is greater than that for sample #1
Correct Answer:
Verified
Q11: The isotope 64Zn has a nuclear radius
Q12: Tritium has a half-life of 12.3 years.What
Q13: If a fossil bone is found to
Q14: Tritium has a half-life of 12.3 years.How
Q15: An ancient building was known to have
Q17: If there are 146 neutrons in 238U,how
Q18: Certain stars at the end of their
Q19: Tritium is radioactive with a half-life of
Q20: A radioactive material initially is observed to
Q21: The half-life of 19N is 0.42 s.What
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents