What is the empirical formula of a compound that is 62.0% C,10.4% H,and 27.5% O by mass?
A) 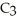
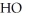
B) 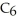
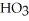
C) 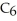
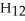
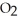
D) 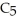
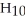
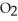
E) 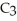
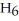
Correct Answer:
Verified
Q128: What is the molar mass of 1-butene
Q129: What is the mass of 0.500 mol
Q130: What mass of ammonia,N H3,contains the same
Q131: What is the mass of a single
Q132: How many moles are there in 3.00
Q134: What mass of phosphorus pentafluoride,PF5,has the same
Q135: A sample of pure calcium fluoride with
Q136: The molecular weight of sucrose ( C12H22O11
Q137: What is the mass of 8.50 ×
Q138: Which of the following has the greatest
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents