What mass of trisodium phosphate is required to prepare 250.0 mL of a solution that is 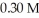
In sodium ion?
A) 37 g
B) 12 g
C) 7.7 g
D) 4.1 g
E) 3.0 g
Correct Answer:
Verified
Q30: What volume of 6.0 M sulfuric acid
Q31: Which of the following processes could theoretically
Q32: A 1.900 g sample of C6H12 is
Q33: 24.0 g of ethane (C2H6)are burned to
Q34: Iron metal reacts with chlorine gas as
Q36: For the reaction symbolized as HCl(aq)+ NaOH(aq)→
Q37: How many mL of 0.024 M solution
Q38: Gases emitted during volcanic activity often contain
Q39: What is the molarity of 10.9 g
Q40: What volume of concentrated acetic acid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents