Using the heat of combustion of methanol as -726.6 kJ/mol and the following data: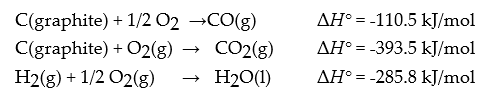
Determine ΔrH° for the following reaction:
CO(g) + 2 H2(g) → CH3OH(l)
A) -349 kJ/mol
B) 157.8 kJ/mol
C) -157.8 kJ/mol
D) 128 kJ/mol
E) -128 kJ/mol
Correct Answer:
Verified
Q90: The standard heat of formation of solid
Q91: Given that ΔfH° [Ag2S(s)] = -32.6 kJ/mole,what
Q92: The heat of combustion,ΔrHcomb,of 1-butene(l),C4H8(l),is -2696.9 kJ/mol.From
Q93: Consider the reaction: Q94: What is the enthalpy of reaction for Q96: Compute ΔrH° for the following reaction.The value Q97: How much heat is involved in the Q98: Given the heat of formation of the Q99: Compute ΔrH° for the following reaction.The value Q100: Given the reactions below,compute ΔrH° for the![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents