Given the heat of formation of the following compounds: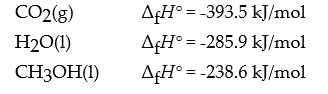
What is the value of ΔrH° for the reaction:
CH3OH(l) + 3/2 O2(g) → CO2(g) + 2 H2O(l)
A) -440.8 kJ/mol
B) -416.9 kJ/mol
C) -1203.9 kJ/mol
D) -346.2 kJ/mol
E) -726.7 kJ/mol
Correct Answer:
Verified
Q93: Consider the reaction: Q94: What is the enthalpy of reaction for Q95: Using the heat of combustion of methanol Q96: Compute ΔrH° for the following reaction.The value Q97: How much heat is involved in the Q99: Compute ΔrH° for the following reaction.The value Q100: Given the reactions below,compute ΔrH° for the Q101: A 5.00 g sample of liquid water Q102: A 50.0 g sample of liquid water Q103: The specific heat capacity of liquid mercury![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents