A 5.00 g sample of copper metal at 25.0 °C is heated by the addition of 96.0 J of energy.The final temperature of the copper is ________ °C.The specific heat capacity of copper is 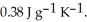
A) 32.3
B) 25.0
C) 25.5
D) 75.5
E) 50.5
Correct Answer:
Verified
Q108: The combustion of titanium with oxygen produces
Q109: How much heat is absorbed when 45.00
Q110: How much heat is absorbed/released when 40.00
Q111: For a particular process that is carried
Q112: At constant pressure,the combustion of 20.0 g
Q114: At 1.0133 bar,the heat of sublimation of
Q115: Calculate the kinetic energy of a 150
Q116: 8.000 moles of H2(g)reacts with 4.000 mol
Q117: The specific heat capacity of solid copper
Q118: For a process at constant pressure,49,600 calories
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents