The specific heat capacity of liquid water is 4.184 J g-1k-1.How many joules of heat are needed to raise the temperature of 6.00 g of water from 36.0 °C to 75.0 °C?
A) 56.0 J
B) 978 J
C) 2.78 × 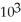 J
J
D) 1.79 × 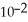 J
J
E) 52.3 J
Correct Answer:
Verified
Q99: Compute ΔrH° for the following reaction.The value
Q100: Given the reactions below,compute ΔrH° for the
Q101: A 5.00 g sample of liquid water
Q102: A 50.0 g sample of liquid water
Q103: The specific heat capacity of liquid mercury
Q105: When 1.50 mol of CH4(g)reacts with excess
Q106: Calculate the work,w,gained or lost by the
Q107: What is the enthalpy change (in kJ)of
Q108: The combustion of titanium with oxygen produces
Q109: How much heat is absorbed when 45.00
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents