Ethanol ( 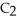
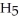 OH) melts at -114 °C.The enthalpy of fusion is 5.02 kJ mol-1.The specific heats of solid and liquid ethanol are 0.97 J g-1 °C-1 and 2.3 J g-1 °C-1,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -50 °C?
OH) melts at -114 °C.The enthalpy of fusion is 5.02 kJ mol-1.The specific heats of solid and liquid ethanol are 0.97 J g-1 °C-1 and 2.3 J g-1 °C-1,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -50 °C?
A) 207.3 kJ
B) -12.7 kJ
C) 6.91 kJ
D) 4192 kJ
E) 9.21 kJ
Correct Answer:
Verified
Q102: The enthalpy change for converting 10.0 g
Q103: Which one of the following would be
Q104: Consider the endothermic reaction:
N2(g)+ O2(g)→ 2 NO(g),ΔrH°
Q105: For a given reaction,ΔrH = -26.6 kJ
Q106: The enthalpy change for converting 1.00 mol
Q108: Consider the reaction: AB(g)? A(g)+ B(g)
Q109: For CO(g)+ H2(g)→ H2CO(g),ΔrH° = -5.36 kJ/mol,and
Q110: Calculate the temperature for which Keq for
Q111: The value of ΔfG at 100.0 °C
Q112: Consider the reaction: AB(g)→ A(g)+ B(g)
(Keq =
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents