Consider the following hypothetical equilibrium reaction:
A2(g) + B2(g) ⇌ 2 AB(g) where Kc = 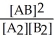
The equilibrium constant for the reaction: 2 A2(g) + 2 B2(g) ⇌ 4 AB(g) is ________.
A) 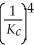
B) Kc4
C) 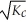
D) 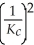
E) Kc2
Correct Answer:
Verified
Q68: For the reaction: 3 Fe(s)+ 4 H2O(g)⇌
Q69: For the reaction: 2 Cl2(g)+ 2 H2O(g)⇌
Q70: Find Kp for the following reaction at
Q71: Write the equilibrium constant expression for the
Q72: For the reaction: 2 SO2(g)+ O2(g)⇌ 2
Q74: At a certain temperature,Kc = 0.0500 and
Q75: Write the equilibrium constant expression for the
Q76: Write the equilibrium constant expression for the
Q77: In the reaction:
2 N2O(g)+ N2H4(g)⇌ 3 N2(g)+
Q78: For 2 NO2(g)⇌ N2O4(g),Kc = [N2O4]/[NO2]2.At equilibrium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents