Dinitrogen tetroxide partially decomposes according to the following equilibrium: 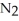
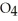 (g) ⇌
(g) ⇌ 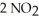 (g)
(g)
A 1.000 L flask is charged with 3.00 × 10-2 mol of 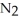
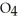 .At equilibrium,2.36 × 10-2 mol of
.At equilibrium,2.36 × 10-2 mol of 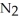
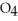 Remains.
Remains. 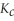 For this reaction is:
For this reaction is:
A) 0.723 mol L-1
B) 0.391 mol L-1
C) 0.212 mol L-1
D) 6.94 × 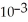 mol L-1
mol L-1
E) 1.92 × 10-4 mol L-1
Correct Answer:
Verified
Q101: For the isomerization reaction:
Butane ⇌ isobutane
Kp equals
Q102: At a certain temperature,nitogen and hydrogen react
Q103: The equilibrium constant Kc is equal to
Q104: For the reaction: N2(g)+ 2 O2(g)⇌ 2
Q105: At a certain temperature,Kc equals 1.40 ×
Q107: Kc is 1.67 × 1020 at 25
Q108: At a certain temperature the equilibrium constant,Kc,equals
Q109: An equilibrium mixture of CO,O2,and CO2 at
Q110: Cyclohexane,C6H12,undergoes a molecular rearrangement in the presence
Q111: The ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents