A 25.0 mL sample of 0.150 mol L-1 aqueous hydrazoic acid is titrated with a 0.150 mol L-1 NaOH(aq) solution.What is the pH after 13.3 mL of base is added?
The 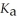 Of hydrazoic acid is 1.9 × 10-5.
Of hydrazoic acid is 1.9 × 10-5.
A) 4.45
B) 1.34
C) 3.03
D) 4.78
E) 4.66
Correct Answer:
Verified
Q116: Calculate the percent ionization of nitrous acid
Q117: What is the pH of a buffer
Q118: Calculate the pH of an aqueous solution
Q119: What is the [CH3COO-]/[CH3COOH] ratio necessary to
Q120: What volume of 5.00 × 10-3 mol
Q122: Sodium hypochlorite,NaOCl,is the active ingredient in household
Q123: What is the approximate pH at the
Q124: Formic acid (HCOOH,Ka = 1.8 × 10-4)is
Q125: What is the pH of the resulting
Q126: What is the pH of a solution
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents