What is the molar solubility of Mg(OH) 2(s) in a basic aqueous solution with a pH of 12.50? Ksp for Mg(OH) 2(s) is 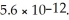
A) 1.8 × 10-10 mol L-1
B) 5.6 × 10-9 mol L-1
C) 2.4 × 10-6 mol L-1
D) 1.1 × 10-4 mol L-1
Correct Answer:
Verified
Q78: Write the solubility product constant for KAl(SO4)2(s)?
A)([K+]
Q79: What is the concentration of Ca2+ in
Q80: The following table lists five compounds and
Q81: In a qualitative cation analysis,the unknown ion
Q82: What is the minimum pH at which
Q84: To a concentrated buffer of pH 4.0
Q85: An aqueous solution contains [Ba2+] = 5.0
Q86: In a qualitative cation analysis,the unknown ion
Q87: Concentrated aqueous solutions of sodium sulfate,ammonium carbonate,and
Q88: What is the molar solubility of barium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents