What is the molar solubility of barium fluoride ( BaF2 ) in water? The solubility-product constant for BaF2 (s) is 1.7 × 10-6 at 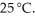
A) 6.5 × 10-4 mol L-1
B) 1.2 × 10-2 mol L-1
C) 1.8 × 10-3 mol L-1
D) 7.5 × 10-3 mol L-1
E) 5.7 × 10-7 mol L-1
Correct Answer:
Verified
Q83: What is the molar solubility of Mg(OH)2(s)in
Q84: To a concentrated buffer of pH 4.0
Q85: An aqueous solution contains [Ba2+] = 5.0
Q86: In a qualitative cation analysis,the unknown ion
Q87: Concentrated aqueous solutions of sodium sulfate,ammonium carbonate,and
Q89: Calculate the solubility (in g L-1)of calcium
Q90: Concentrated aqueous solutions of iron (III)chloride,sodium hydroxide,and
Q91: In which of the following aqueous solutions
Q92: Calculate the molar solubility of thallium chloride
Q93: In a qualitative cation analysis,the unknown ion
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents