Solid potassium chromate (K2CrO4) is slowly added to a solution containing 0.50 mol L-1 AgNO3(aq) and 0.50 mol L-1 Ba(NO3) 2(aq) .What is the Ag+(aq) concentration when BaCrO4(s) just starts to precipitate?
The Ksp for Ag2CrO4(s) and BaCrO4(s) are 1.1 × 10-12 and 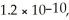 Respectively.
Respectively.
A) 6.5 × 10-5 mol L-1
B) 1.3 × 10-4 mol L-1
C) 3.2 × 10-4 mol L-1
D) 6.8 × 10-2 mol L-1
Correct Answer:
Verified
Q94: What ratio of [NH4+]/[NH3] would provide a
Q95: Concentrated aqueous solutions of copper (I)nitrate,sodium sulfate
Q96: Saturated aqueous solutions of sodium sulfate,ammonium carbonate,and
Q97: What is the molar solubility of manganese
Q98: In a qualitative cation analysis,the unknown ion
Q99: A swimming pool was sufficiently alkaline so
Q100: Calculate the Ksp for silver carbonate if
Q101: What is the molar solubility of AgCl(s)in
Q102: A solution of NaF(aq)is added dropwise to
Q103: What is the molar solubility of AgCl
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents